
There are unique considerations regarding the patentability of certain chemical inventions. In this article, we look at the case law regarding claims that are distinguished from the prior art solely by their chemical purity, their enantiomeric form, and their polymorph structure.
Chemical Purity
Chemical Purity generally refers to a measurement of the amount of impurities found in a chemical sample. A sample with a purity of 100% would mean the sample contains only one molecule or compound, the sample containing no impurities or side-products.
In both Europe and the UK, it was previously held that any document disclosing a method of making a low molecular weight chemical compound anticipated the production of this molecule in all grades of purity unless evidence was provided to the contrary (see T 990/96). Because methods of purifying such compounds are well-known, it was considered that all purities were considered implicitly disclosed. In order to establish novelty, the burden of proof was therefore on the applicant to demonstrate that conventional methods of obtaining a claimed level of purity of compound had failed.
However, a more recent case in Europe, T 1085/13, departed from this view. In this case, it was reasoned that a claimed purity is only disclosed implicitly if the method of preparing the compound described in the prior art inevitably results in the level of purity as claimed. If a different purification method was used or a further purification is required, the claim should be considered novel over the prior art.
However, in order to be patentable, the claim should also be held to involve an inventive step. To assess this, it is considered whether the skilled person could arrive at a compound having the claimed higher purity using conventional techniques in the art, such as recrystallisation, distillation and chromatography.
With regards to the facts of the particular case in T 1085/13, the claimed compound having a purity of at least 99.5% was held to be novel over the prior art because it was convincingly demonstrated that the purification method disclosed in D1 only resulted in a purity of 97.91%. The claim was also held to involve an inventive step because the skilled person encountered technical difficulties when trying to isolate the compound from undesired by-products.
This suggests the assessment of patentability of compounds having a certain level of purity could be patentable in Europe if sufficient evidence is provided to show that prior art methods fail to disclose compounds having said purity, and where achieving an improved level of purity is shown to be technically challenging.
Considerations for Drafting Patent Applications relating to compositions or compounds with improved purity.
- Provide evidence in the application as filed, preferably as comparative evidence, demonstrating that obtaining a high level of purity is not possible using conventional methods.
- Specify the novel level of purity in the claim (e.g., a purity of at least 95 wt.%).
- If possible, identify the impurities that are present when the compound is purified using routine or conventional methods. Discuss the steps taken to remove these impurities, including technical considerations and reasoning. Finally, consider specifying these impurities in the claims and/or include fallback positions in the description which specify that these impurities are present in the sample below a certain level (e.g., less than 1 wt.%).
Enantiomers
In chemistry, compounds having a chiral centre (i.e., an asymmetric carbon atom) can exist as optical isomers, each isomer being a mirror image of the other. A mixture of enantiomers is usually referred to as a “racemic mixture”. Naming convention is that chiral centres, and enantiomers, are referred to as being either “R” or “S”, which is determined using the Cahn-Ingold-Prelog (CIP) priority rules.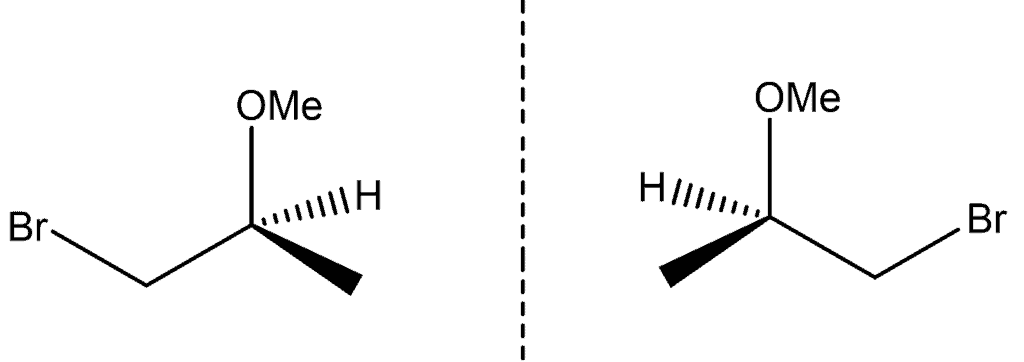 According to T 296/87 , a disclosure of a racemic mixture of enantiomers did not anticipate the novelty of each enantiomer that formed the mixture. Thus, isolating and claiming either the “R” or “S” enantiomer of a compound is considered to be novel over a prior disclosure that only discloses a racemic mixture.
According to T 296/87 , a disclosure of a racemic mixture of enantiomers did not anticipate the novelty of each enantiomer that formed the mixture. Thus, isolating and claiming either the “R” or “S” enantiomer of a compound is considered to be novel over a prior disclosure that only discloses a racemic mixture.
However, case law both in the UK and Europe suggests that the enantiomer of a compound, where the racemic mixture is known, would typically not be held to be inventive, since resolving enantiomers from a racemic compound is often an obvious or a routine step (see UK Case – Novartis AG v Generics (UK) Limited and T 296/87 ).
T 296/87, for example, indicates that it is well-known that a compound of pharmaceutical activity may have one enantiomer that has a pharmaceutical activity greater than the other enantiomer or the racemic mixture (see Reasons, 8.4.1). However, the conclusions of this case acknowledge that a lack of obviousness would not be found in all instances. An inventive step could be acknowledged, for example, “when a different result is achieved”(see Reasons, 8.4.2). This suggests that an inventive step could be acknowledged if one resolved enantiomer has a surprising technical effect or different properties which would not be evident from the properties of the racemic mixture. The Board also acknowledged that an inventive step may be acknowledged if a compound had more than one chiral centre, where the number of possible isomers multiplying exponentially ”(see Reasons, 8.4.2).
In the UK, there is also precedent that an exception to a lack of inventive step may include situations where it has been difficult to prepare the enantiomers by standard resolution techniques (see UK Case – Generics (UK) Limited v H Lundbeck A/S). Such an approach appears to be consistent with established case law in Europe (see T595/90).
It therefore appears possible that a claim to a particular enantiomer may be considered patentable in certain circumstances.
Considerations for Drafting Patent Applications relating to specific enantiomers
- Provide evidence in the application as filed demonstrating that one enantiomer has a particularly surprising or different properties compared with the other enantiomer(s) or the racemic mixture.
- If difficulties were encountered when separating the two enantiomers, provide evidence that standard resolution methods could not be used, and provide detailed discussion of the specific method steps taken to spatially resolve the enantiomer in question.
Polymorphism
In chemistry, polymorphism refers to the existence of more than one form or crystal structure of a solid material, each form referred to as a “polymorph”. A solid chemical compound may be, for example, in an amorphous or crystalline form. Crystalline forms may be further characterized by having a particular crystal structure or crystal habit. These features can be defined and distinguished from the prior art using spectroscopy.
Case law indicates that a particular polymorph of a compound is considered to be novel over the prior art if the prior art does not provide an enabling disclosure of a particular polymorphic form. This includes any disclosure that inevitably results in the formation of that particular polymorph, even if this is not stated explicitly in the prior disclosure (see UK case – Synthon BV v Smithkline Beecham plc [2005] UKHL 59).
However, merely claiming the crystalline form of a compound is unlikely to be considered inventive if the advantage is one that is expected to arise. In T 1555/127, the Board reasoned that “the mere provision of a crystalline form is not regarded as involving an inventive step. Investigation of whether active compounds are prone to crystalline transformation and characterisation of such crystalline forms is routine practice in the pharmaceutical industry”. Similarly, in T 0777/08, a crystalline polymorph having improved drying and filterability was considered obvious over an amorphous form because these characteristics were considered to be generally expected from the crystalline forms of all compounds.
However, an inventive step has been acknowledged in other cases where the new polymorph has an unexpected property. For example, in T 1422/12, a specific polymorph characterised by peaks in a powder XRD pattern was found to be more stable than the amorphous polymorph with respect to epimerisation, wherein a reduction of epimerisation resulted in improved biological activity (see Reasons 2.3.3 and 2.7).
Considerations for Drafting Patent Applications relating to a new polymorph of a known product
- For novelty, provide evidence and data in the application as filed demonstrating that the polymorph is new over prior disclosure. This should ideally be demonstrated in terms of crystallographic/IR data/NMR data/melting point data. Certain distinguishing characteristics of this data (e.g., the presence of certain peaks in spectra) should ideally be claimed.
- For inventive step, provide comparative data showing that there is a surprising technical effect associated with a particular polymorph as compared with previously known polymorphs. Evidence suggesting one particular polymorph is advantageous over a large number of polymorphs, including various crystalline polymorphs, is also more likely to be persuasive.
Our Chemistry and Life Sciences team are always on hand to advise you, or to identify pitfalls, in patenting in this area. Feel free to reach out to us with any questions you might have.
This is for general information only and does not constitute legal advice. Should you require advice on this or any other topic then please contact hlk@hlk-ip.com or your usual Haseltine Lake Kempner advisor.
